Moxifloxacin Injection 1.6 mg/mL freeflex® Bag 250 mL Label Change Notice
October 23, 2019
Dear Valued Customer,
Fresenius Kabi Canada would like to advise all customers of our product label transition for Moxifloxacin Injection 1.6 mg/mL freeflex® Bag 250 mL. Colour has been added to the overwrap label, and the font size for the product name has been increased. Please note there has been no change to the formulation and the product remains latex, preservative, PVC, and DEHP free.
The new labelled product will be available as of November 1, 2019. Please note during the transition period, the existing product with old labels may still be in distribution until inventory is depleted. Therefore, you may receive both the old and new product for a brief period of time.
A more detailed look at the “old” versus “new” labelling can be seen below.
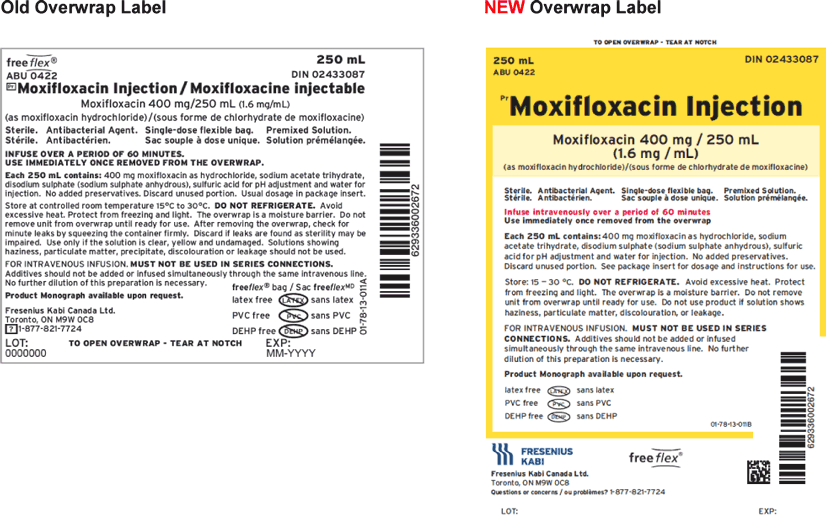
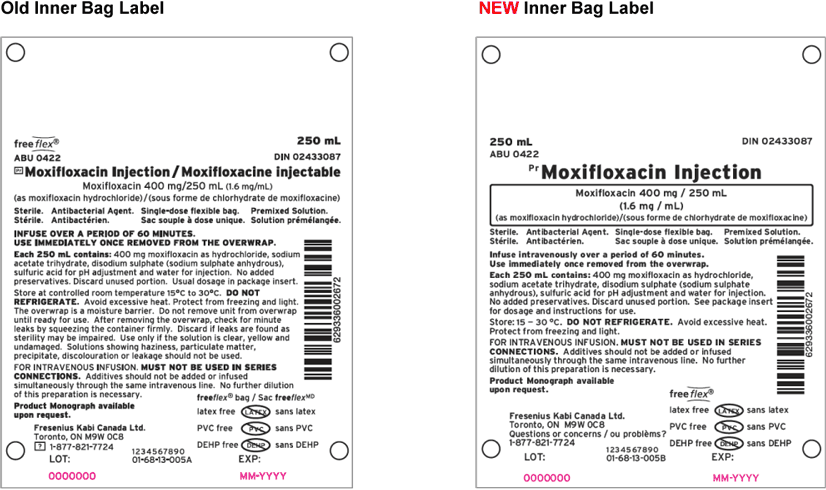
We would like to assure you that Fresenius Kabi Canada is dedicated to providing products that meet the highest quality standards.
If you would like to obtain more information on these products, please contact your hospital representative or our Customer Service Department at (877) 821-7724.
Sincerely,
George Shamsoun
Senior Manager – Marketing, IV Drugs
george.shamsoun@fresenius-kabi.com
