Glycopyrrolate Injection, USP Now Available from Fresenius Kabi
Fresenius Kabi now offers 0.2 mg per mL, 0.4 mg per 2 mL, 1 mg per 5 mL and 4 mg per 20 mL presentations
July 18, 2018
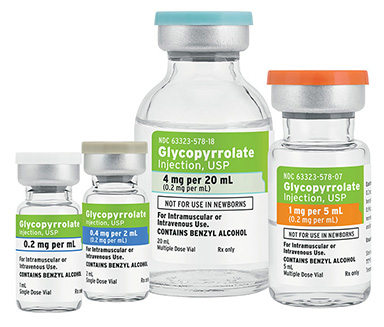
LAKE ZURICH, Ill., July 18, 2018 – Fresenius Kabi announced today the immediate availability in the United States of Glycopyrrolate Injection, USP in 0.2 mg per mL, 0.4 mg per 2 mL, 1 mg per 5 mL and 4 mg per 20 mL presentations.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company is a leading developer, manufacturer and provider of injected and infused medicines in the United States with special expertise in producing high quality, affordable generic alternatives to more expensive brand-name drugs.
“With the introduction of our Glycopyrrolate offerings Fresenius Kabi is pleased to expand its portfolio of anesthesia products, bringing affordable options and additional product supply to hospitals and clinics,” said John Ducker, president and CEO of Fresenius Kabi USA.
About Glycopyrrolate Injection, USP
INDICATIONS AND USAGE
Anesthesia: Glycopyrrolate Injection, USP is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free acidity of gastric secretions; and to block cardiac vagal inhibitory reflexes during induction of anesthesia and intubation. When indicated, Glycopyrrolate Injection may be used intraoperatively to counteract surgically or drug induced or vagal reflexes associated arrhythmias.
Peptic Ulcer: For use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or when oral medication is not tolerated.
IMPORTANT SAFETY INFORMATION
Glycopyrrolate Injection, USP is contraindicated in patients with known hypersensitivity to glycopyrrolate or any of its inactive ingredients.
In the management of peptic ulcer patients, because of the longer duration of therapy, Glycopyrrolate Injection may be contraindicated in patients with the following concurrent conditions: glaucoma; obstructive uropathy; obstructive disease of the gastrointestinal tract; paralytic ileus, intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
Glaucoma: use Glycopyrrolate injection with great caution, if at all.
Benzyl Alcohol: Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The practitioner must consider the daily metabolic load of benzyl alcohol from all sources.
Drowsiness and Blurred Vision: Use caution while performing activities requiring mental alertness.
Heat prostration: May cause decrease sweating.
Incomplete intestinal obstruction: Diarrhea may be an early symptom. In patients with an ileostomy or colostomy, Glycopyrrolate Injection, may be inappropriate and possibly harmful.
Tachycardia: May increase heart rate, investigate any tachycardia before giving glycopyrrolate.
Use with caution in patients with: coronary artery disease; congestive heart failure; cardiac arrhythmias; hypertension; hyperthyroidism.
Renal impairment: Use with caution in patients with renal disease since the renal elimination of glycopyrrolate may be severely impaired in patients with renal failure.
Use Glycopyrrolate with caution in the elderly and in all patients with autonomic neuropathy, hepatic disease, ulcerative colitis, prostatic hypertrophy, or hiatal hernia since anticholinergic drugs may aggravate these conditions.
The use of anticholinergic drugs in the treatment of gastric ulcer may produce a delay in gastric emptying due to antral statis.
Adverse effects related to anticholinergics may include: Xerostomia, urinary hesitancy and retention; blurred vision and photophobia due to mydriasis (dilation of the pupil); cycloplegia; increased ocular tension; tachycardia; palpitation; decreased sweating; loss of taste; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling. Severe allergic reactions include: Anaphylactic/anaphylactoid reactions; hypersensitivity; urticaria, pruritus, dry skin, and other dermal manifestations; some degree of mental confusion and/or excitement, especially in elderly persons.
Adverse events reported from post-marketing experience with glycopyrrolate include: malignant hyperthermia; cardiac arrhythmias (including bradycardia, ventricular tachycardia, ventricular fibrillation); cardiac arrest; hypertension; hypotension; seizures, respiratory arrest, injection site reactions including pruritus, edema, erythema, and pain. Heart block and QTc interval prolongation associated with the combined use of glycopyrrolate and an anticholinesterase have been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Glycopyrrolate Injection, USP safely and effectively. Please click on the following link (https://tinyurl.com/yc5hkg3a) for the full prescribing information for Glycopyrrolate Injection, USP at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.
