Fresenius Kabi Launches Posaconazole Injection for Prevention or Treatment of Fungal Infections
January 29, 2024
Produced in the U.S., the generic anti-fungal drug is fully substitutable for Noxafil®
LAKE ZURICH, Ill., January 29, 2024 – Fresenius Kabi announced today it has introduced Posaconazole Injection, a generic substitute for Noxafil®, for use in treating or preventing serious fungal infections in adults and children who have an increased chance of getting these infections due to a weakened immune system. Now available in the U.S., Posaconazole Injection is the newest addition to Fresenius Kabi’s portfolio of more than 30 anti-infective molecules.
Available in a 300 mg per 16.7 mL vial, Posaconazole Injection is used to treat invasive aspergillosis, a serious fungal infection, in adults and teenagers 13 years of age and older. It is also used to prevent invasive aspergillus and candida infections in patients who are severely immunocompromised, including adults and pediatric patients age 2 or older.
“The introduction of Posaconazole Injection reflects Fresenius Kabi’s ongoing commitment to expanding our portfolio of affordable generic options for the treatment and/or prevention of life-threatening infections,” said John Ducker, president and CEO of Fresenius Kabi USA.
Posaconazole Injection is produced at the company’s pharmaceutical production site in Grand Island, New York. Fresenius Kabi has invested nearly $1 billion to expand and update its U.S. pharmaceutical production and distribution network. More than 70 percent of the product units shipped in the U.S. by Fresenius Kabi are drugs listed on the FDA’s Essential Medicines List.
“We are doubling our U.S. manufacturing capacity to enhance the resilience of our supply chain, which helps assure fast, reliable service for our customers and the patients they serve,” said Lindsey Thomas, senior vice president of marketing of Fresenius Kabi USA. “Our investments will help patients gain access to the treatments they need – when and where they need them most.”
Learn more about Fresenius Kabi’s commitment to strengthening its domestic supply chain at MoreInAmerica.com.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in injectable medicines, biosimilars, and technologies for infusion, transfusion, and clinical nutrition. The company’s products and services are used to help care for patients with critical and chronic conditions. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/join-us and follow us on LinkedIn.
References
*Noxafil® is a registered trademark of Merck Sharp & Dohme LLC.
About Posaconazole Injection
Posaconazole is an azole antifungal indicated as follows:
- Posaconazole injection is indicated for the treatment of invasive aspergillosis in adults and pediatric patients 13 years of age and older.
- Posaconazole is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy as follows:
- Posaconazole injection: adults and pediatric patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
Posaconazole is contraindicated in persons with known hypersensitivity to posaconazole or other azole antifungal agents.
Coadministration of Posaconazole with the following drugs is contraindicated; Posaconazole increases concentrations and toxicities of:
- Sirolimus
- CYP3A4 substrates (pimozide, quinidine): can result in QTc interval prolongation and cases of torsades de pointes (TdP)
- HMG-CoA Reductase Inhibitors Primarily Metabolized through CYP3A4
- Ergot alkaloids
- Venetoclax: in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) at initiation and during the ramp of phase
Calcineurin-Inhibitor Toxicity: Posaconazole increases concentrations of cyclosporine or tacrolimus; reduce dose of cyclosporine and tacrolimus and monitor concentrations frequently.
Arrhythmias and QTc Prolongation: Posaconazole has been shown to prolong the QTc interval and cause cases of TdP. Administer with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs known to prolong QTc interval and metabolized through CYP3A4.
Electrolyte Disturbances: Monitor and correct, especially those involving potassium (K+), magnesium (Mg++), and calcium (Ca++), before and during Posaconazole therapy.
Hepatic Toxicity: Elevations in liver tests may occur. Discontinuation should be considered in patients who develop abnormal liver tests or monitor liver tests during treatment.
Renal Impairment: Posaconazole injection should be avoided in patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Posaconazole injection.
Concomitant Use with Midazolam: Posaconazole can prolong hypnotic/sedative effects. Monitor patients and benzodiazepine receptor antagonists should be available.
Vincristine Toxicity: Concomitant administration of azole antifungals, including Posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions; reserve azole antifungals, including Posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options.
Venetoclax Toxicity: Concomitant administration of Posaconazole with venetoclax may increase venetoclax toxicities, including the risk of tumor lysis syndrome, neutropenia, and serious infections; monitor for toxicity and reduce venetoclax dose.
Adult Patients: Common adverse reactions in studies with Posaconazole in adults are diarrhea, nausea, fever, vomiting, headache, coughing, and hypokalemia.
Pediatric Patients: Common adverse reactions (incidence >20%) receiving 6 mg/kg Posaconazole injection in a study in pediatric patients are pyrexia, febrile neutropenia, vomiting, mucosal inflammation, pruritus, hypertension, hypokalemia, and stomatitis.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
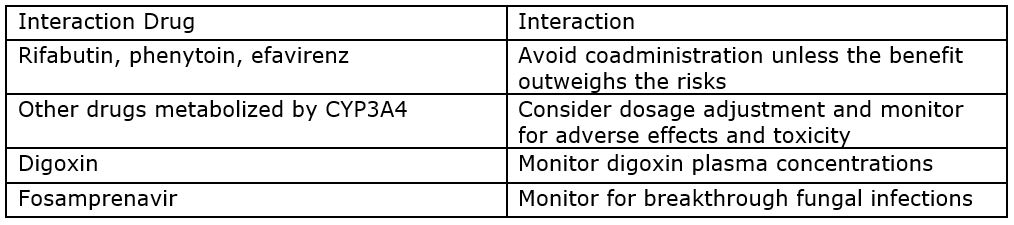
Drug Interactions:
Pregnancy: Based on animal data, may cause fetal harm.
Pediatrics: Safety and effectiveness in patients younger than 2 years of age have not been established.
Severe Renal Impairment: Monitor closely for breakthrough fungal infections.
This Important Safety Information does not include all the information needed to use Posaconazole Injection safely and effectively. Please see the full prescribing information for Posaconazole Injection available at www.fresenius-kabi.com/us.
###
