Fresenius Kabi Introduces Pralatrexate Injection for the Treatment of Relapsed or Refractory Peripheral T-cell Lymphoma
Improving access to oncology therapies in the United States
December 8, 2022
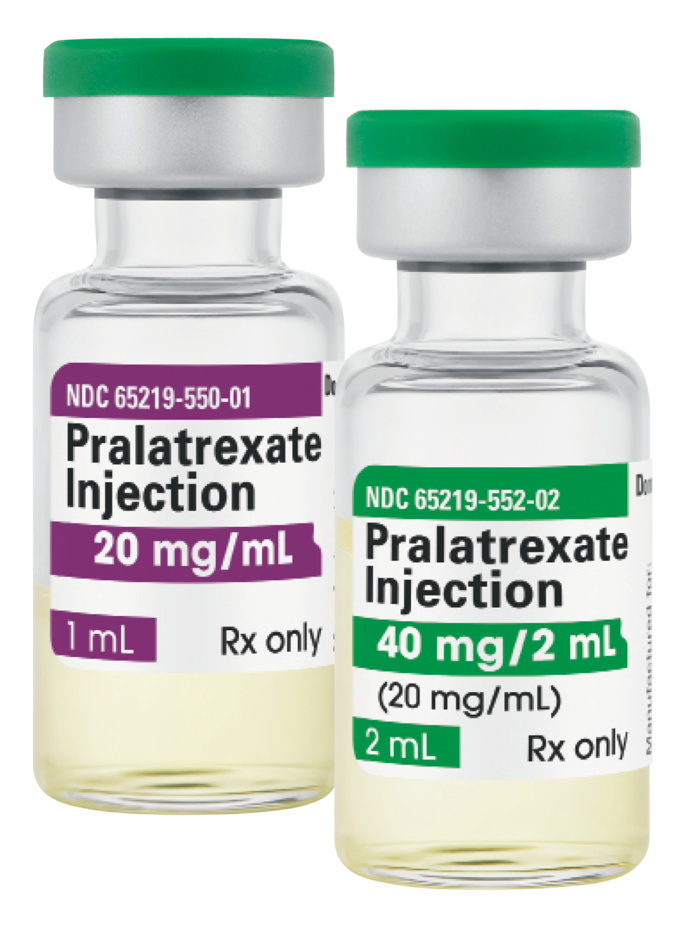
LAKE ZURICH, Ill., December 8, 2022 – Fresenius Kabi announced today it has introduced Pralatrexate Injection, a generic equivalent to Folotyn®, for the treatment of relapsed or refractory peripheral T-cell lymphoma. Fresenius Kabi Pralatrexate Injection is available immediately in the United States and is the newest addition to the company’s injectable oncology medicine portfolio, the largest in U.S. health care.
“Fresenius Kabi is committed to expanding access to high-quality, high-value oncology medicines,” said John Ducker, president and CEO of Fresenius Kabi USA. “We are pleased to offer Pralatrexate in the United States and we intend to continue to bring new oncology medicines, including biosimilars, to U.S. patients.”
Fresenius Kabi Pralatrexate Injection provides clinicians and patients with a generic treatment option for adult patients with relapsed or refractory peripheral T-cell lymphoma. Fresenius Kabi Pralatrexate Injection is available in two single-dose vial presentations: 20 mg/1 mL and 40 mg/2 mL.
About Pralatrexate Injection
Pralatrexate injection is a dihydrofolate reductase inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL).
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Important Safety Information
Myelosuppression: Monitor complete blood counts and omit and/or reduce dose based on ANC and platelet count.
Mucositis: Monitor at least weekly. Omit and/or reduce dose for grade 2 or higher mucositis.
Dermatologic reactions: Reactions, including fatal reactions, occurred and may be progressive and increase in severity with further treatment. Monitor closely and withhold or discontinue Pralatrexate injection based on severity.
Tumor lysis syndrome: Monitor patients who are increased risk and treat promptly.
Hepatic toxicity: Monitor for liver function tests. Omit until recovery, adjust or discontinue therapy based on severity.
Risk of increased toxicity with renal impairment: Avoid Pralatrexate injection in patients with end stage renal disease with or without dialysis. If the potential benefit of administration justifies the potential risk, monitor renal function and reduce the Pralatrexate injection dose based on adverse reactions.
Embryo-fetal toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use an effective method of contraception.
Most common adverse reactions (>35%) are mucositis, thrombocytopenia, nausea, and fatigue.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800‐551‐7176, option 5, or FDA at 1‐800‐FDA‐1088 or www.fda.gov/medwatch.
Avoid coadministration with probenecid or nonsteroidal anti-inflammatory drugs. If coadministration is unavoidable, monitor for increased risk of adverse reactions.
Lactation: Advise not to breastfeed.
This Important Safety Information does not include all the information needed to use Pralatrexate Injection safely and effectively. Please see full prescribing information for Pralatrexate Injection at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion, and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/join-us and follow us on LinkedIn.
###
*Folotyn® is a registered trademark of Acrotech Biopharm
