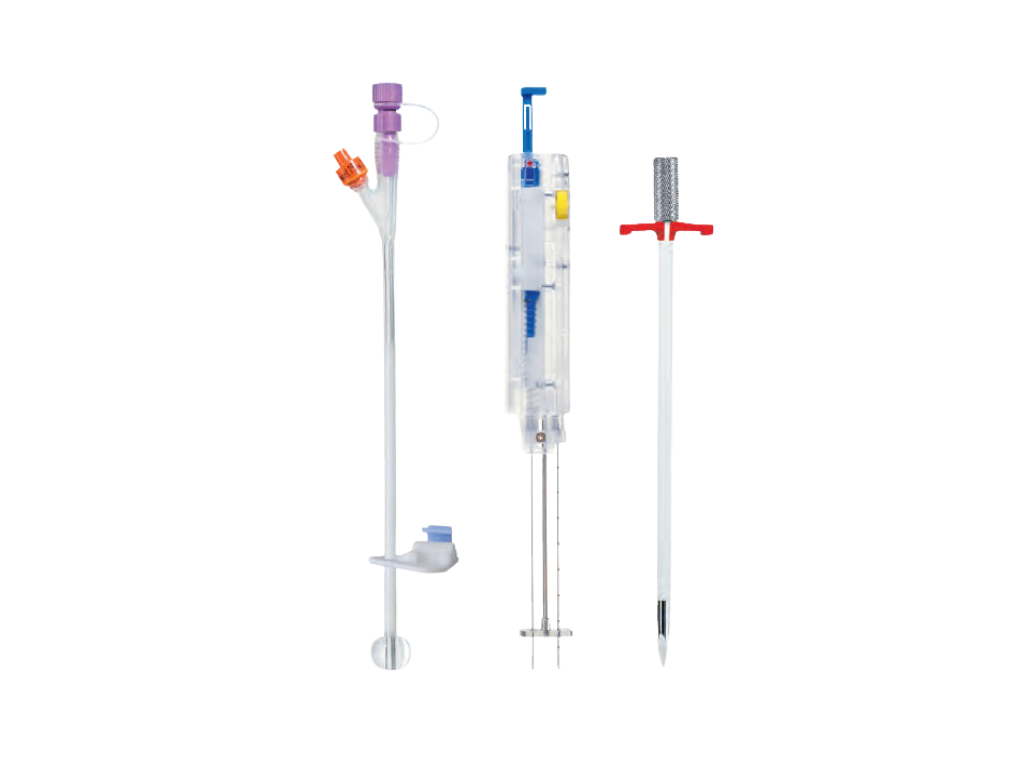
- CH/FR 15
- Shaft length: 23 cm +/- 1cm
- External diameter: 5mm
- Connector: ENFit
Freka® Pexact II
Details
- Description
- Duration of use
- Features
- Indication
- Presentation and order codes
Balloon tube with 90 day lifespan. Can be changed to a Freka GastroTube four weeks after placement within a fully formed and healed stoma.
- Latex free silicone tube, with retention balloon
- Gastropexy device with suturing material, single handed operation
- Improved fiaxation device enables single handed operation
- Trocar with peel away sheath, CH/FR 16
- Suturing material
- Scalpel
- Balloon tube with 90 day lifespan
- Can be changed to Freka GastroTube four weeks after placement, within a fully formed and healed stoma
- Balloon fill volume - 5ml
Long-term intragastric feeding and gastric decompression, especially for patients where a PEG cannot be placed by pull technique such as:
- severe stenosis of the oesophagus
- oesophageal varices
- oesophageal diverticula
- corrosive burns in the upper gastrointestinal tract
- tumours in the ENT area - tube placed before surgical procedure
- radiotherapy or surgery of the upper gastrointestinal tract
- previous operations on the mouth or pharynx
- only nasal endoscopy possible
Absolute contraindications
- no transillumination and positive needle aspiration test
- blood-clotting disorders
- severe general wound-healing disorders
- sepsis
- peritonitis
- acute pancreatitis
- obstructions in the lower gastrointestinal tract
- tumour infiltration at the puncture site
- ulceration at pucture site
- visible liver tumour
Relative contraindictions (to be decided case-by-case)
- immune suppression
- ascites
- peritoneal carcinosis
- peritoneal dialysis (PD)
- ventriculoperitoneal shunt
- severe pschoses
- anorexia nervosa
Product | Pack Size | Product Code |
| Freka Pexact II (CH/FR 15) | 1 | 7601365 |
Freka® Pexact II Placement Video
GB-PEG-2400009 October 2024
For Healthcare Professionals Only