Our approach to biosimilars is simple, but our work is complex: making advanced, life-changing biologic medicines more accessible to more patients. Fresenius Kabi leverages our global expertise to develop and produce biosimilars.
Biopharma
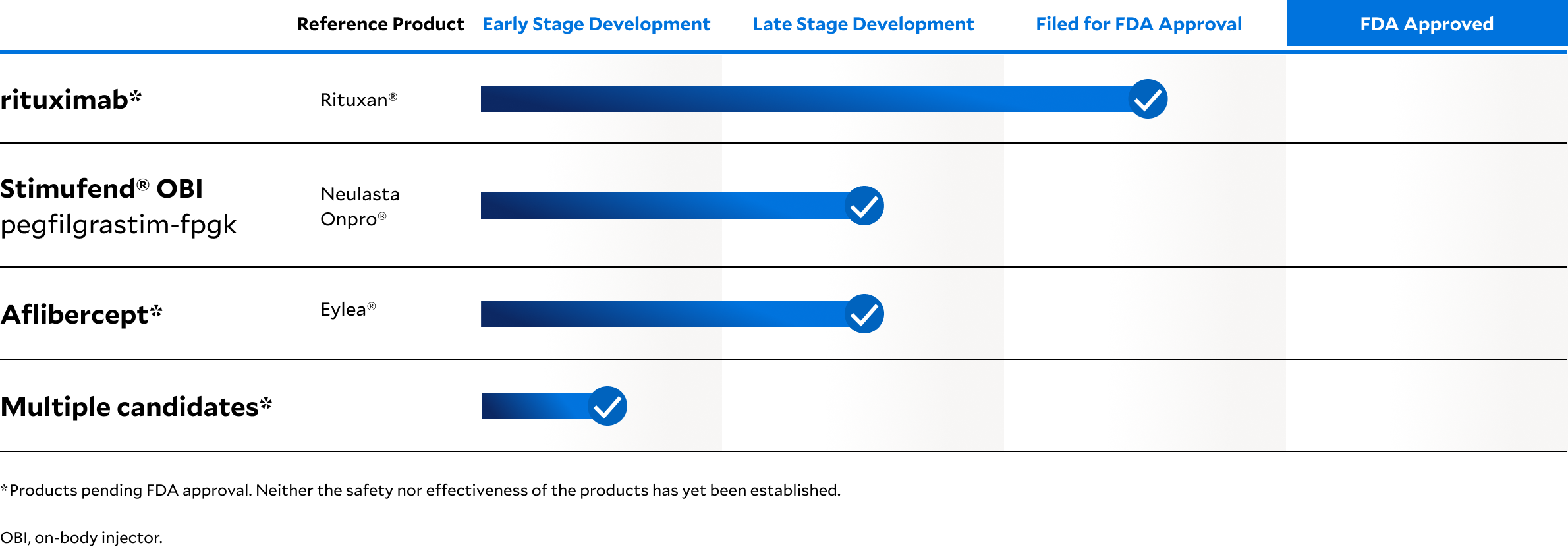
Dedicated to bringing biosimilar medicines to the world

The Fresenius Kabi Biosimilars Pipeline Reflects Our Commitment to Patients
We continuously expand our product pipeline—launching biosimilars in more markets around the globe while optimizing our manufacturing, supply, and commercial capabilities together with our partners.
Explore the growing Fresenius Kabi biosimilars pipeline targeting mechanisms within immunology, oncology, hematology, and osteoporosis: