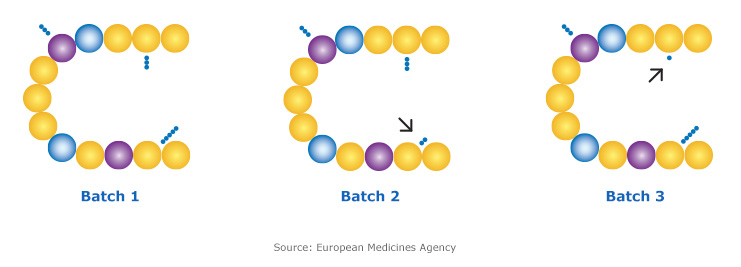
Differences between product batches
Biologic medicines are made in “batches”. During the manufacturing process, variability between different batches of the same biosimilar molecule is expected, and is carefully monitored by regulatory authorities such as Health Canada. These acceptable, within-product differences are not known to affect safety, efficacy or patient outcomes.2
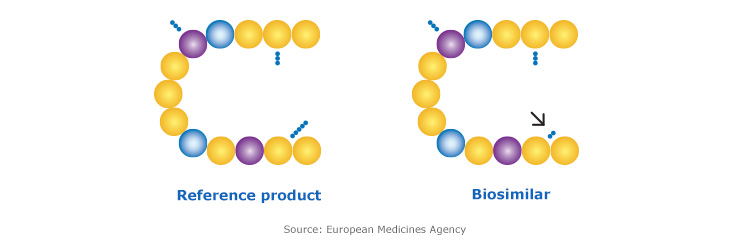
How biosimilars are approved in Canada
Health Canada has developed a robust, science-based framework for authorizing biosimilars. Biosimilars must be manufactured to the same regulatory standards as other biologic drugs and authorized after scientific evaluation by Health Canada.1
Health Canada requires that biosimilar manufacturers demonstrate the similarity of their biosimilar to the reference biologic drug via a step-wise approach:1
- Beginning with structural and functional studies
- Continuing with human clinical studies
Biosimilar manufacturers must independently demonstrate the quality of their product and perform comparative studies to demonstrate highly similar structure, function, efficacy and safety to the reference biologic.1
Learn more about choosing a biosimilar
References:
1.Health Canada. Biosimilar biologic drugs in Canada: fact sheet. 2019.
2. European Medicines Agency (EMA). Biosimilars in the EU: Information guide for healthcare professionals. 2017. Accessed June 26, 2020 at: www.ema.europa.eu.

