- Linezolid is a synthetic antibiotic of oxazolidine class and active against most Gram-positive bacteria.
- It acts a protein synthesis inhibitor stopping the growth of bacteria by disrupting their production of proteins (bacteriostatic action).
- The exact mechanism of action of linezolid appears to be unique in that it blocks the initiation of protein production, and not one of the later steps.
Kabizolid
- Features
- Indication
- Dosage
- Productinfo
KABIZOLID l.V. lniection is indicated in the treatment of the following infections caused by susceptible strains of the designated microorganisms:
- Vancomycin-resistant Enterococcus faecium infections, including cases with concurrent bacteremia.
- Nosocomial pneumonia caused by Staphylococcus aureus (methicillin-susceptible and resistant strains), 'or Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP]).
- Complicated skin and skin structure infections, including diabetic foot infections, without concomitant osteomyelitis, caused by Staphylococcus aureus (methicillinsusceptibleand-resistant strains), Streptococcus pyrogenes, or Streptococcus agatactiae.
- Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible only) or Streptococcus pyogenes.
- Community-acquired pneumonia caused by Streptocaccus pneumoniae (including multidrug resistant strains [MDRSP]), including cases with concurrent bacteremia, or Staphylococcus aureus (methicillin-susceptible strains only).
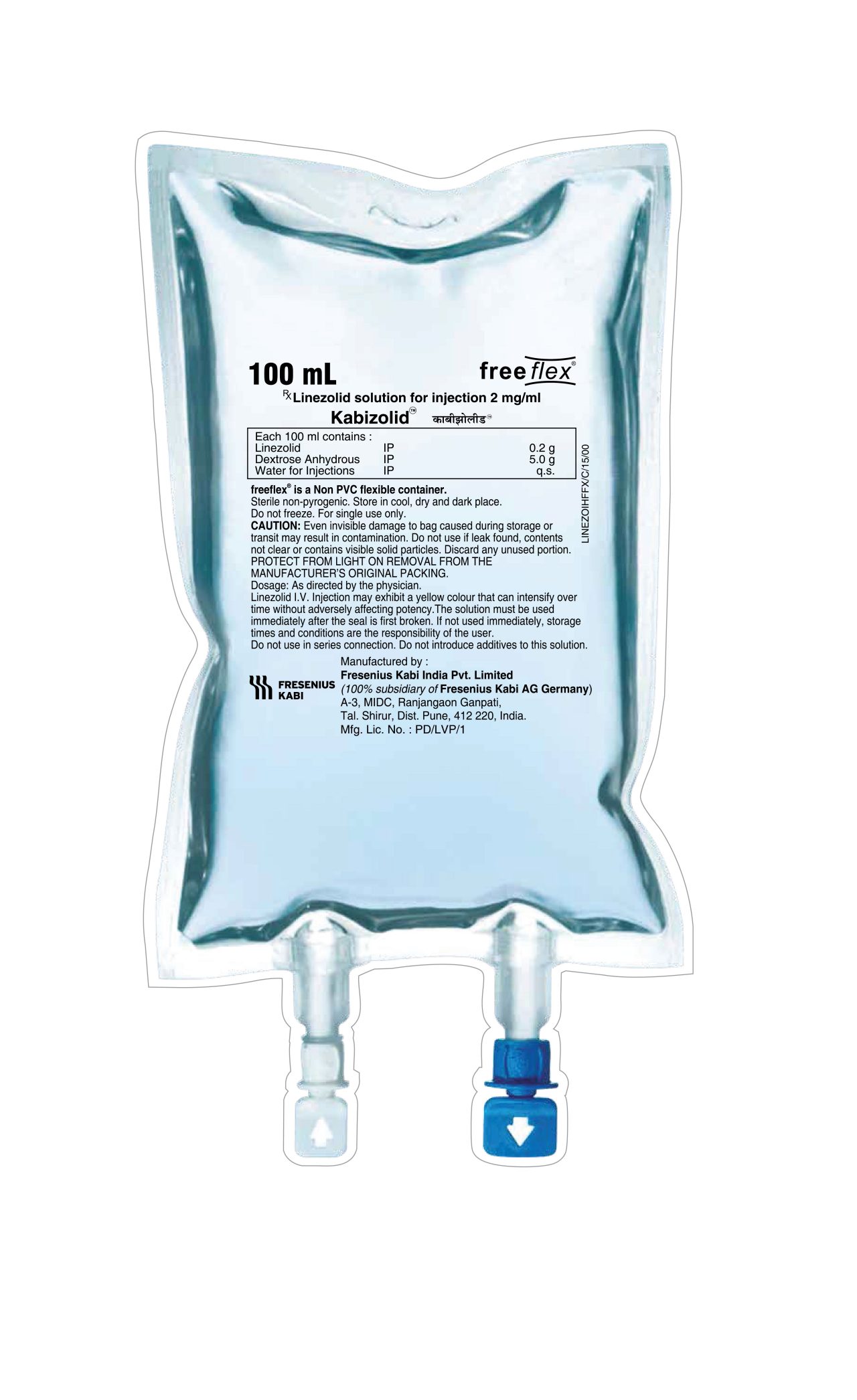
Packaging: KABIZOLID I.V. Injection Freeflex bag of 100 ml and 300 ml
| Dosage and Route of Administration | |||
| INDICATION | PEDIATRIC PATIENTS (BIRTH THROUGH 11 YEARS OF AGE) | ADULTS AND ADOLESCENTS (12 YEARS AND OLDER) | RECOMMENDED DURATION OF TREATMENT (CONSECUTIVE DAYS) |
| Nosocomial pneumonia | 10 mg/kg iv or oral every 8 hours | 600 mg iv or oral every 12 hours | 10 to 14 |
| Community-acquired pneumonia, including concurrent bacteremia | 10 mg/kg iv or oral every 8 hours | 600 mg iv or oral every 12 hours | 10 to 14 |
| Complicated skin and skin structure infections | 10 mg/kg iv or oral every 8 hours | 600 mg iv or oral every 12 hours | 10 to 14 |
| Vancomycin-resistant Enterococcus faecium infections, including concurrent bacteremia | 10 mg/kg iv or oral every 8 hours | 600 mg iv or oral every 12 hours | 14 to 28 |
| Uncomplicated skin and skin structure infections | Less than 5 years: 10 mg/kg iv or oral every 8 hours, 5 - 11 years: 10 mg/kg iv or oral every 12 hours | Adults: 400 mg every 12 hours, Adolescents: 600 mg oral every 12 hours | 10 to 14 |
- Linezolid solution for injection 2mg/ml
Each 100 ml contains
Linezolid IP 0.2 g
Dextrose (Anhydrous) IP 5.0 g
Water for Injections IP q.s.