- Kabidarba comes with Preventis technology
Kabidarba
- Features
- Indication
- Dosage
- Packaging
- Productinfo
- Darbepoetin alfa injection is indicated for the treatment of anemia with CKD including patients on dialysis and patients not on dialysis.
- Darbepoetin alfa is administered subcutaneously or intravenously as a single weekly injection.
- Correction of Anemia: For patients with CKD on dialysis ≥1 year of age, the initial dose by subcutaneous or intravenous administration is 0.45mcg/kg body weight, as a single injection once weekly. Alternatively, in patients not receiving dialysis, an initial dose of 0.75 mcg/kg may be administered subcutaneously or intravenously as a single injection once every 2 weeks or 1.5 mcg/kg once monthly.
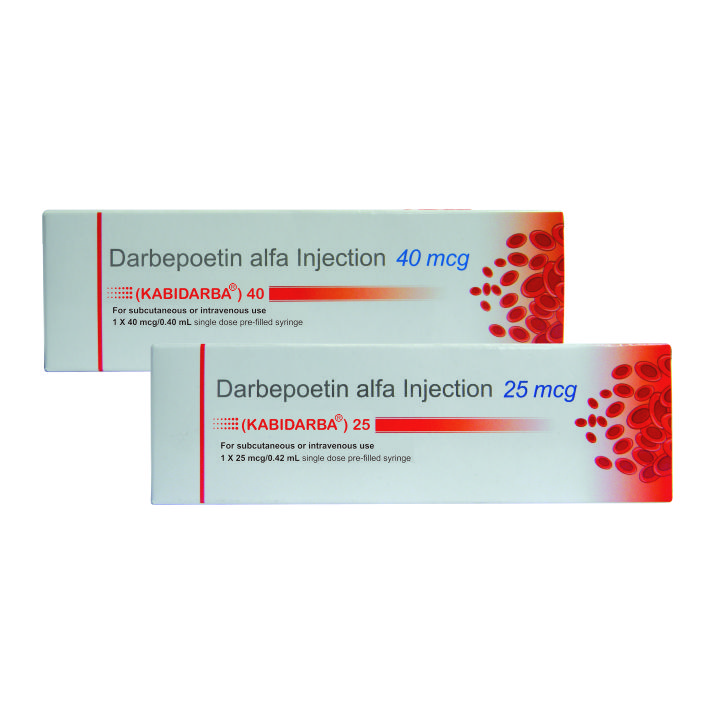
- Packaging:
1 X 25 mcg/0.42 ml single dose PFS
1 X 40 mcg/0.40 ml single dose PFS
- Darbepoetin alfa is available in 25 and 40 mcg dose strengths. 25mcg/0.42 ml and 40mcg/0.40ml.
- Each dose contains excipients viz., Polysorbate 80, Sodium phosphate monobasic monohydrate, Sodium phosphate dibasic anhydrous and Sodium chloride in Water for injection with pH 6.2±0.2.