Fresenius Kabi Introduces Levothyroxine Sodium Injection Solution
First-and-only FDA-approved solution formulation of this medication now available in 100 mcg per 5 mL, 200 mcg per 5 mL and 500 mcg per 5 mL single-dose vials
July 8, 2019
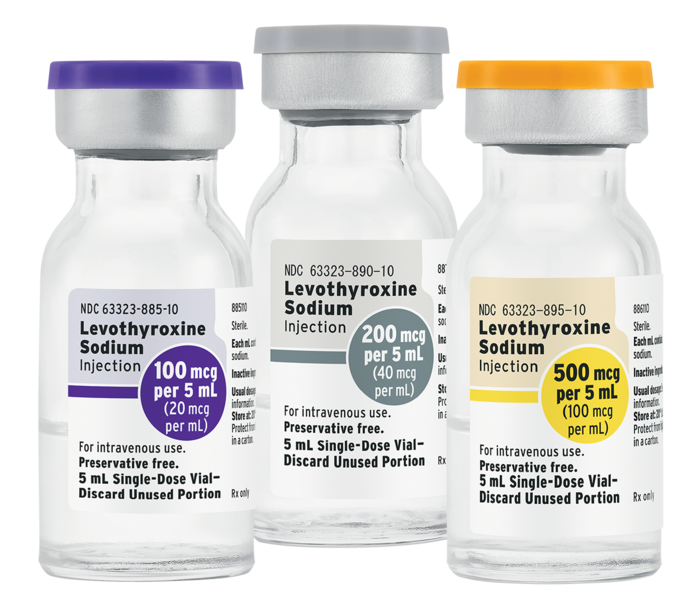
LAKE ZURICH, Ill., July 8, 2019 – Fresenius Kabi announced today the immediate availability in the United States of Levothyroxine Sodium Injection Solution in 100 mcg per 5 mL, 200 mcg per 5 mL and 500 mcg per 5 mL single-dose vials.
Fresenius Kabi Levothyroxine Sodium Injection is the first-and-only FDA-approved solution formulation for this medicine available in the U.S. Fresenius Kabi also sells Levothyroxine Sodium for Injection as a lyophilized powder in 100 mcg, 200 mcg and 500 mcg per 5 mL vial presentations.
“Fresenius Kabi is pleased to continue to increase the number of products we offer to help care for patients with critical illnesses, including life-threatening thyroid conditions,” said John Ducker, president and CEO of Fresenius Kabi USA. “This new liquid formulation of Levothyroxine promotes safe medication practices and operational efficiency by eliminating the need for reconstitution.”
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. To learn more about the company’s expanding centers for U.S. manufacturing and innovation, including career opportunities, please visit us at www.fresenius-kabi.com/us.
INDICATIONS AND USAGE
Levothyroxine Sodium Injection is L-thyroxine (T4) indicated in adult patients for the treatment of myxedema coma.
Limitations of Use:
Not recommended as a substitute for oral levothyroxine sodium because the relative bioavailability of Levothyroxine Sodium Injection to oral levothyroxine sodium has not been established and there is a risk of inaccurate dose conversion.
IMPORTANT SAFETY INFORMATION

Levothyroxine Sodium Injection is contraindicated in uncorrected adrenal insufficiency.
Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease: Overtreatment may cause arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death, particularly in patients with cardiovascular disease and in elderly patients. Start with lower doses in elderly patients and in patients with underlying cardiovascular disease and monitor patients after administration.
Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency: Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment.
Worsening of Diabetic Control: May worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control.
Adverse reactions associated with Levothyroxine Sodium Injection are primarily those of hyperthyroidism due to therapeutic overdosage: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating, headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia, tremors, muscle weakness, muscle spasm, palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest, dyspnea, diarrhea, vomiting, abdominal cramps, elevations in liver function tests, flushing and rash.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and target tissue response) that may alter the therapeutic response to Levothyroxine Sodium Injection.
This Important Safety Information does not include all the information needed to use Levothyroxine Sodium Injection safely and effectively. Please click on this link (https://tinyurl.com/y3kmhe2b) for the full prescribing information for Levothyroxine Sodium Injection. Full prescribing information is also available at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.
